Page 58 - Annual Report_21-22
P. 58
Adduct-based p-doping of organic semiconductors
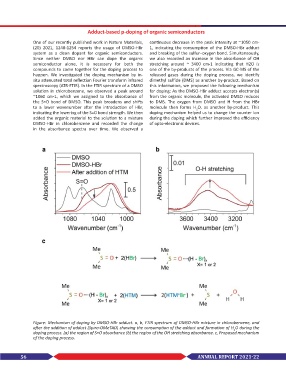
One of our recently published work in Nature Materials, continuous decrease in the peak intensity at ~1050 cm-
(20) 2021, 1248-1254 reports the usage of DMSO-HBr 1, indicating the consumption of the DMSO-HBr adduct
system as a clean dopant for organic semiconductors. and breaking of the sulfur–oxygen bond. Simultaneously,
Since neither DMSO nor HBr can dope the organic we also recorded an increase in the absorbance of OH
semiconductor alone, it is necessary for both the stretching around ~ 3400 cm-1 indicating that H2O is
compounds to come together for the doping process to one of the by-products of the process. Via GC-MS of the
happen. We investigated the doping mechanism by in- released gases during the doping process, we identify
situ attenuated total reflection Fourier transform infrared dimethyl sulfide (DMS) as another by-product. Based on
spectroscopy (ATR-FTIR). In the FTIR spectrum of a DMSO this information, we proposed the following mechanism
solution in chlorobenzene, we observed a peak around for doping: As the DMSO-HBr adduct accepts electron(s)
~1060 cm-1, which we assigned to the absorbance of from the organic molecule, the activated DMSO reduces
the S=O bond of DMSO. This peak broadens and shifts to DMS. The oxygen from DMSO and H from the HBr
to a lower wavenumber after the introduction of HBr, molecule then forms H O, as another by-product. This
2
indicating the lowering of the S=O bond strength. We then doping mechanism helped us to change the counter ion
added the organic material to the solution to a mixture during the doping which further improved the efficiency
DMSO-HBr in chlorobenzene and recorded the change of opto-electronic devices.
in the absorbance spectra over time. We observed a
Figure: Mechanism of doping by DMSO-HBr adduct. a, b, FTIR spectrum of DMSO-HBr mixture in chlorobenzene, and
after the addition of adduct (Spiro-OMeTAD) showing the consumption of the adduct and formation of H O during the
2
doping process. (a) the region of S=O absorbance (b) the region of the OH stretching absorbance. c, Proposed mechanism
of the doping process.
56 ANNUAL REPORT 2021-22